33.04.01 Industrial pharmacy
The Master's program is aimed at training specialists in management and organization of the development, research, production, regulation and use of medicines.
This program provides for the training of qualified personnel for the pharmaceutical industry, mainly pharmaceutical, chemical pharmaceutical, and biopharmaceutical enterprises.
This program provides for the training of qualified personnel for the pharmaceutical industry, mainly pharmaceutical, chemical pharmaceutical, and biopharmaceutical enterprises.
Level of education:
Master’s degree
Form of training:
Full-time (day department)
Venue of training:
Moscow
Entrance exams:
— Chemistry
Programs, specializations:
Industrial pharmacy
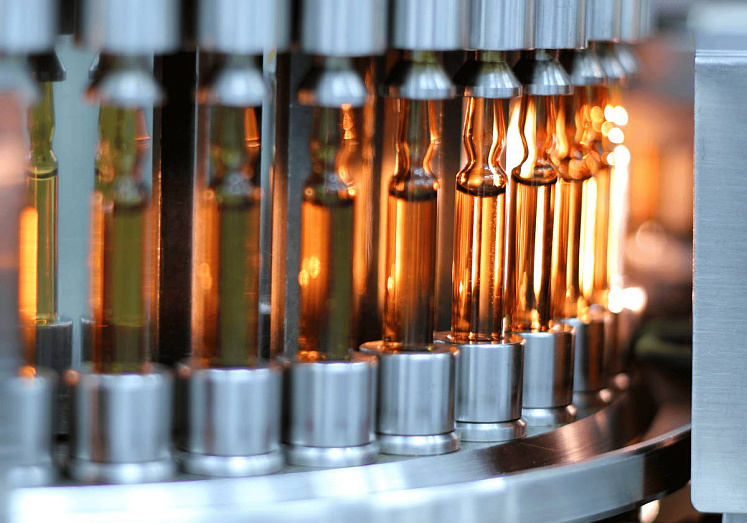
In the course of training, students receive theoretical knowledge and practical skills in the organization, planning and management of on-going pharmaceutical, chemical-pharmaceutical, biotechnological processes, and chemical production.
The program provides for the development of skills for working with academic documentation and technical specifications and regulations for the production of pharmaceutical substances and finished dosage forms; conducting of pharmaceutical and chemical-pharmaceutical processes and industries in conformity with legal and regulatory national and international acts; organization and implementation of quality control of raw materials, semi- and finished products. Due to the organized specialized departments at the University, students can take practical training courses and internships at industrial facilities of partner organizations.
The topics of the master’s theses are both of academic and practical significance and are done on the request of partner enterprises under the guidance of experienced faculty members of the department. Thus, starting from the first year, students have a chance to interact with potential employers while engaged in current research.
The program provides for the development of skills for working with academic documentation and technical specifications and regulations for the production of pharmaceutical substances and finished dosage forms; conducting of pharmaceutical and chemical-pharmaceutical processes and industries in conformity with legal and regulatory national and international acts; organization and implementation of quality control of raw materials, semi- and finished products. Due to the organized specialized departments at the University, students can take practical training courses and internships at industrial facilities of partner organizations.
The topics of the master’s theses are both of academic and practical significance and are done on the request of partner enterprises under the guidance of experienced faculty members of the department. Thus, starting from the first year, students have a chance to interact with potential employers while engaged in current research.
Alumni can be employed as
- process engineers (biotechnologists)
- registration specialists
- specialists in pharmaceutical production validation
- specialists in the organization of scientific research
- specialists of the quality control department
Program subjects
- Life cycle of a medicinal product
- Medicines quality assurance system
- Technology of finished dosage forms
- Validation of pharmaceutical production
- Formulation development
- Bioethics
- Preclinical research and clinical trials
- Legal acts and regulations for the creation and organization of medicinal drug production
- Pharmacovigilance
- Fundamentals of drug production organization
- Microbiology and industrial sanitation in pharmaceutical production
- API production technologies
- Standardization of raw materials of plant origin
- Standardization and quality control of medicines
Department of Biotechnology and Industrial Pharmacy
- Educational Activity
-
Institutes
- Institute of Information Technologies
-
Institute of Artificial Intelligence
- About the Institute
- Institute Administration
- History of the Institute
-
Training programs
- Bachelor's Degree Programs
-
Master's Degree Programs
- 01.04.02 Applied mathematics and information science
- 09.04.01. Informatics and computer engineering
- 12.04.04 Biotechnical systems and technologies
- 15.04.04 Automation of technological processes and production
- 15.04.06 Mechatronics and robotics
- 27.04.03 System analysis and management
- 27.04.04 Engineering system control
- Infrastructure
- Alumni
- Contacts
- Institute for Cybersecurity and Digital Technologies
-
Institute for Advanced Technologies and Industrial Programming
- About the Institute
- Institute Administration
- History of the Institute
-
Training programs
-
Bachelor's Degree Programs
- 09.03.02 Information systems and technologies
- 11.03.04 Electronics and nanoelectronics
- 12.03.05 Laser technology and laser techniques
- 15.03.01 Mechanical engineering
- 22.03.01 Materials science and technology
- 27.03.01 Standardization and metrology
- 28.03.01 Nanotechnology and microsystems engineering
- 29.03.04 Decorative material working techniques
- 54.03.01 Graphic design
-
Master's Degree Programs
- 09.04.02. Information systems and technologies
- 11.04.04 Electronics and nanoelectronics
- 12.04.02 Optical engineering
- 15.04.01 Mechanical engineering
- 22.04.01 Materials science and technology
- 27.04.01 Standardization and metrology
- 29.04.04 Decorative material working techniques
- 54.04.01 Graphic design
-
Bachelor's Degree Programs
- Infrastructure
- Alumni
- Contacts
- Institute of Radio Electronics and Informatics
- Institute of Management Technologies
- Lomonosov Institute of Fine Chemical Technologies
- Institute of International Education
-
Mega-Laboratories
- Motion Capture Laboratory
- Immersive Technologies Laboratory
- Laboratory for the Development and Transfer of Microfluidic Technologies (DTMT)
- Cell Technologies Megalaboratory operating on the basis of the Department of Chemistry and Technology of Biologically Active Compounds, Medical and Organic Chemistry named after N.A. Preobrazhensky
- General Biotechnology Megalaboratory
- Industry 4.0: Digital Robotized Production center
- Laboratory of Intelligent Autonomous and Multi-Agent Robotic Systems
- Research and Educational Center for Biosynthesis, Isolation and Purification of Monoclonal Antibodies (Generium)
- Rare and Precious Metals Research and Technological Center operating on the basis of the Department of Chemistry and Technology of Rare Elements named after K.A. Bolshakov
- Laboratory of Analytic, Modeling, Design and Digital Prototyping Technologies
- Import Substitution of Information Technologies Educational and scientific testing complex
- Smart Production Systems Educational and Scientific Center
- Elastomers. Thermoplastics. Technologies Educational and Research Center operating on the basis of the Department of Chemistry and Technology of Elastomer Processing named after F.F. Koshelev
- Catalytic and Mass Exchange Processes center
- Center of Innovative Technologies in Microelectronics
- Center for Cybersports Robotics
- Mobile Robotics University Laboratory
- Radio electronic Technologies Megalaboratory
- Departmental Situation Center of the Ministry of Science and Higher Education of the Russian Federation for monitoring the sphere of education and science
- Scientific and Educational Center for Medical Radiology and Dosimetry
- Laboratory of Geographic Information Systems and Technologies
- Educational and Research Center for Space Monitoring ("CosMoCenter")
- Additive Polymer Technologies Center
- Cyber Threat Research Megalaboratory
- Digital Center of Rosatom State Corporation
- Laser Technologies Megalaboratory
- Mathematical Modeling and Artificial Intelligence Megalaboratory
- Megalaboratory of Digital and Additive Technologies in Mechanical Engineering
- Nanomaterials and Nanostructures Diagnostics Center
- Techno-coworking
- TESLA Educational and Research Center
- Bachelor's Degree Programs
- Master's Degree Programs
- Additional Education Programs
- Physical Education and Sports
© 2026 MIREA - Russian Technological University




